
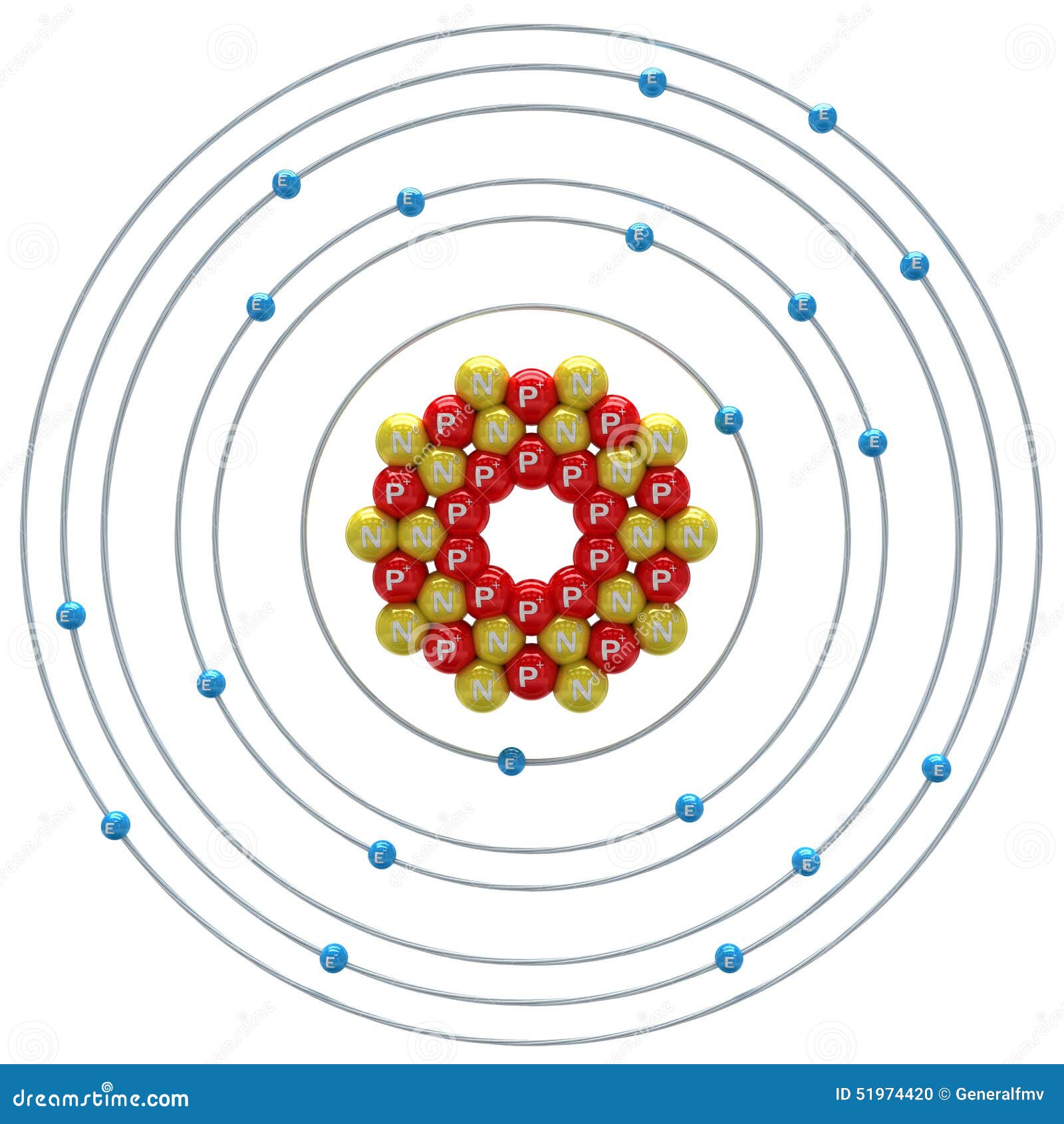
The compound composed of these ions exhibits properties entirely different from the properties of the elements sodium and chlorine. They write new content and verify and edit content received from contributors. The cookies is used to store the user consent for the cookies in the category "Necessary". Individual atoms can gain or lose electrons. Zamani (ESA/Webb), and the PDRs4All ERS Team. They are formed when elements ESA/Webb, NASA, CSA, M. Write the electron configurations of these cations. ex: oxygen, atomic number 8 and How is it possible for mantle rock to flow? What effects accomplishments did Francisco have. What are answers of bbc compacta class9 module 1? The formula Na2Cl2 also has balanced charges, but the convention is to use the lowest ratio of ions, which would be one of each. The cookie is used to store the user consent for the cookies in the category "Other. It requires 769 kJ of energy to dissociate one mole of solid NaCl into separate gaseous Na+ and Cl ions: When forming a cation, an atom of a main group element tends to lose all of its valence electrons, thus assuming the electronic structure of the noble gas that precedes it in the periodic table. PTAA is a better candidate for hole transport compar Journal of Materials Chemistry A Emerging Investigators Cations (positively-charged ions) and anions (negatively-charged ions) are formed when a metal loses electrons, and a nonmetal gains those electrons. Accessibility Cations are smaller than the parent atom because they've lost electrons. It has one valence electron in the third principal energy level. How do cations form? What does please be guided accordingly phrase means? Anions are atoms or molecules with a negative charge due to a higher ratio of electrons. For example, when chlorine takes an electron from sodium, and sodium gives that electron to chlorine, they become ions and form NaCl. Do they have to give members warning before they bar you? Surface-applied thiol affects the perovskite work function it ameliorates hole injection into the Spiro overlayer, thus improving device performance. Representative Metals, Metalloids, and Nonmetals, Chapter 19. Which elements will form cations? How can you tell is a firm is incorporated? Transition and inner transition metal elements behave differently than main group elements. has 2,8,1 and it losses 1 electron to get duplet confg. Which of the following atoms would be expected to form negative ions in binary ionic compounds and which would be expected to form positive ions: Br, Ca, Na, N, F, Al, Sn, S, Cd? An atom becomes unstable because of the number of neutrons. PMC Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. Introductory Chemistry - 1st Canadian Edition by Jessie A. However, there has been a push to reduce the amount of sodium most people ingest every day: avoid processed and manufactured foods, read labels on packaged foods (which include an indication of the sodium content), dont oversalt foods, and use other herbs and spices besides salt in cooking. The sodium ion is isoelectronic with the neon atom. Table 3.4 Some Sodium Compounds Added to Food is a partial list of some sodium additives used in food. Unable to load your collection due to an error, Unable to load your delegates due to an error.
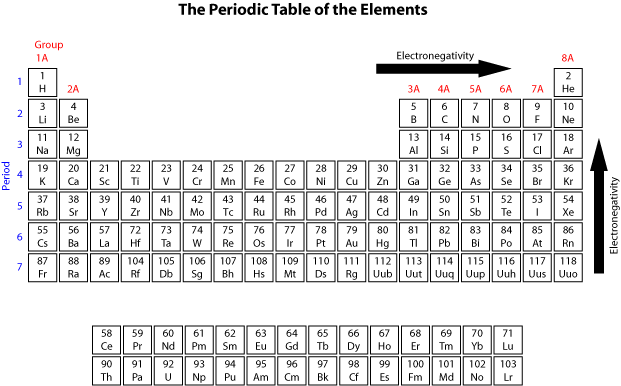
Cobalt is another element that can form more than one possible charged ion (2+and 3+), while lead can form 2+or 4+cations.


 0 kommentar(er)
0 kommentar(er)
